Guidelines for CBD When Patients Are Using Medications Metabolized by the P450 System
Are there any guidelines for clinicians or patients regarding the safe use of CBD with patients on medications metabolized by the P450 system? (Question from the SCC Members Forum)
Patricia Frye, MD: I tell my patients to take CBD an hour apart from most everything since patient’s medications change in between visits. For those known to utilize CYP450 3A4, and have narrow therapeutic windows, like statins, warfarin, AED’s particularly clobazam and valproic acid, beta blockers, tricyclics, MAOIs, I recommend 2-3 hour spacing. It might be overkill, but that’s what I do.
Jeff Hergenrather, MD: This is a good and reasonable question without a clear and conclusive answer. Considering that Drs. Sulak, Sareto, and Goldstein published a 2017 paper on the use of artisanal cannabis and epilepsy, these doctors may offer better advice than I can offer.
I think the key here is making dose adjustments to these drugs when high doses of CBD are being used.
I am not in primary care so I’m not ordering the blood levels of AEDs, warfarin, or other drugs. I have always asked patients for a complete medication list at the first and each subsequent visit. Suffice to say, I am not finding reports of significant drug/drug interactions with cannabis and the pharmaceuticals used in thousands of patients. Of most concern would be clobazam, but other drugs may warrant blood level testing as well.
Many medications and compounds are metabolized by the Cytochrome P450 metabolic pathway. What is clear is that these pathways vary from person to person, so determining a simple answer is not really possible. What is also clear to me is that the presence of “usual doses of cannabis” do not seem to overload the metabolic pathways. So even if a drug and cannabis are competing for the same pathway (3A4, 2C19, etc) the liver is able to handle the metabolism of both without untoward effects. The important variables being hepatic insufficiency and high doses of cannabis, especially high dose cannabidiol.
Is it also important to consider: what is a high dose of cannabidiol?
The less people know about cannabis medicine the more conservative and cautious are the directives about the use of CBD. Ten years ago Ethan Russo, MD and I were saying that there are no significant drug-drug interactions between cannabis and any pharmaceutical. As clobazam (Onfi®) came into more common use as an anti-epileptic drug, an infant in Seattle with a difficult seizure disorder had a disastrous effect while taking both clobazam and high dose CBD in an effort to control seizures. The drug/drug interaction caused the metabolite of clobazam, desclobazam, to reach very high levels which caused excessive sedation and loss of consciousness. I believe that CBD was stopped resulting in intractable seizures that resulted in the child’s death. I never saw this case report published so I don’t even know if the story was accurate.
Since then there have been assertions that ANY drug that is metabolized by the same pathways as cannabis could and would cause abnormal blood levels of pharmaceuticals. I think this is an oversimplification of the matter and not backed up by clinical results.
In the table published by Devinsky et al. in 2014 about the effect of CBD on the blood levels of common antiepileptic drugs (AEDs), there was a rather compelling alteration of AED mean blood levels. What was never clear from this work was the dose of CBD being utilized (see the table below). As a consequence of these findings, I’ve simply advised patients who take a regular, daily dose of these medicines to have their prescribing physician check the blood levels for these drugs and adjust their dosing accordingly.
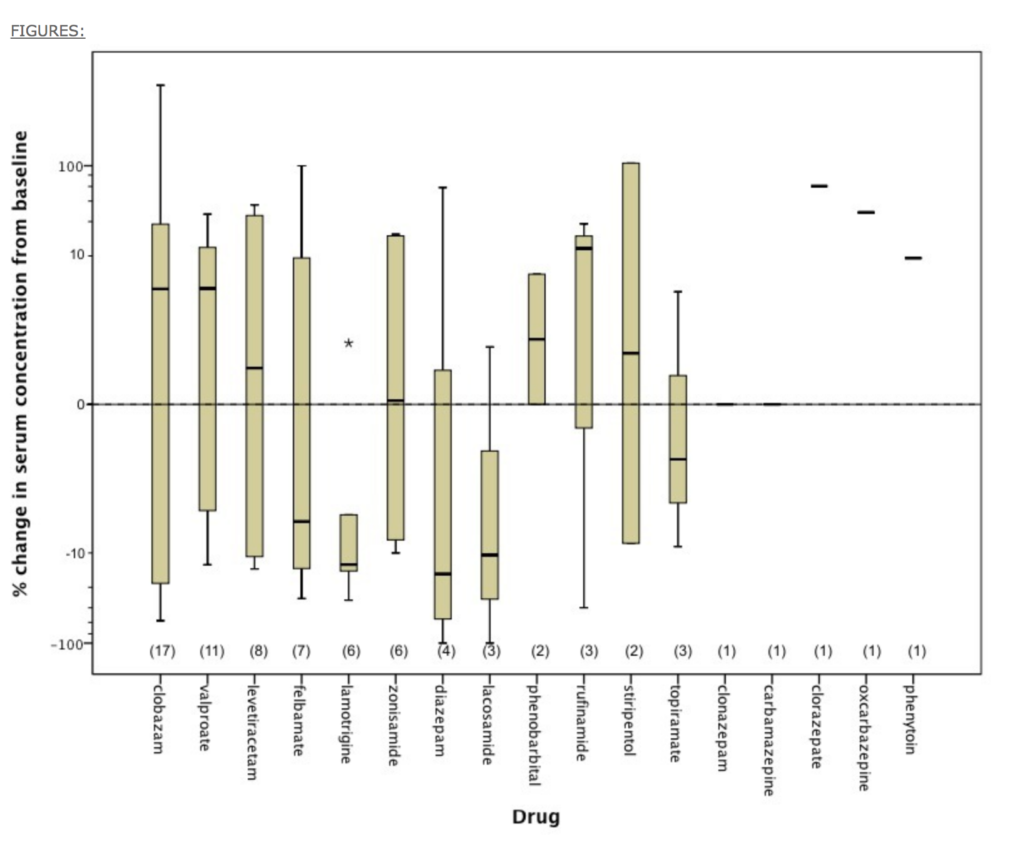
THE EFFECT OF EPIDIOLEX (CANNABIDIOL) ON SERUM LEVELS OF CONCOMITANT ANTI-EPILEPTIC DRUGS IN CHILDREN AND YOUNG ADULTS WITH TREATMENT-RESISTANT EPILEPSY IN AN EXPANDED ACCESS PROGRAM
Other medications may be problematic with high doses of CBD. I believe (but don’t have evidence) that a dose of greater than 1 mg/kg/day warrants investigation with blood testing of various pharmaceuticals. I refer the patient back to the prescribing physician for a lab requisition for blood level testing.
Patricia Frye, MD: I agree. The Epidiolex trials were using CBD doses of 20mg/kg. I don’t think many are using that dosage with pediatric seizure patients, except for maybe neurologists. Most patients are given 0.25-5mg/kg of CBD, and some a little higher. But even at these lower doses, we can see changes in plasma levels with clobazam and valproate. The pediatric oncologists here are happy to check chemotherapeutic plasma levels and adjust doses accordingly.
This exchange was taken from the SCC Members Forum, used to discuss pressing inquiries related to cannabis in clinical practice. The SCC Members Forum is the place to explore and connect with experienced healthcare professionals and get direct answers to questions. Learn more about membership perks or contact us for further information.